Characterisation of sorbents for CO2 adsorption
Research line developed within the CEEP (Centro Eccellenza Energia Pulita) project on the topic of low-carbon energy production.
Date:
16 September 2021
Among the various strategies currently being studied for carbon dioxide capture, the use of solid sorbents is particularly promising, as well as being cost-effective. The technique with solid sorbents refers to gas-solid reactions (as in the case of the carbonation reaction by calcium oxide) or chemical and physical adsorption. However, several issues, including complicated preparation methods, low adsorption capacities, not always feasible regenerations and instability under certain conditions, still call into question its widespread use in industrial-scale applications.
Sotacarbo's research activity, developed in collaboration with London South Bank University, focuses on two-dimensional boron nitride (BN) and doped boron nitride (BNMe) nanosheet material with different transition metal loadings.
Porous boron nitrides exhibit high surface areas, high microporous volumes, bond polarity and high thermal stability, presenting themselves as potential candidates for a wide range of applications in gas adsorption: CO2 capture, hydrogen storage, formaldehyde removal from air and paraffin/olefin separation. Furthermore, their mechanical properties and high thermal conductivity make them very promising as a new class of adsorbent materials.
However, the mechanism of adsorption is not always entirely clear, and understanding it, in addition to modifying the porous properties, can enable a significant improvement in gas adsorption capacity. To increase the CO2 adsorption capacity of BN materials, it is essential to modify their electronic and porous structure. A method that seems to be effective is the incorporation of heteroatoms into the structure.
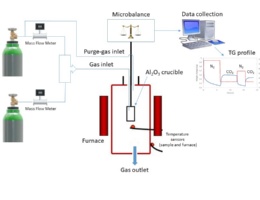
The main goal of the activity is therefore the evaluation of the post-combustion CO2 adsorption capacity and thermal stability of various low-cost high-performance solid adsorbents by means of thermogravimetric analysis.
To investigate the suitability of the synthesised materials as regenerable solid adsorbents for continuous use, several adsorption/desorption cycles were performed under various operating conditions. Indeed, regenerability and reversibility in the process plays a key role in the final choice of sorbent. Direct processing of the experimental data made it possible to determine the mass adsorption of the sample (dry adsorbent) during CO2 adsorption and subsequently the CO2capacity. This study demonstrates the applicability of metal-free and metal-doped modifications of BN for increased pure CO2 capacity.
The study can be extended by economic analysis to assess the industrial feasibility of materials and identify key critical areas for optimisation.
Last update
06/11/2024, 12:03